Background
Diagen Bioinnovation Inc.

Established: June 9, 2023
Core Beliefs:
- Establish international competitive advantage of Taiwan’s own bio-related technologies and brands
- Combining Taiwan’s advantages in ICT and biotechnology industries across fields; integrating nanochip technology and molecular diagnosis
- Epidemic outbreak control as early as possible; expand epidemic prevention capacity; avoid impacting the country’s emergency and serious medical capacity
- Help dealing with the technical difficulties of facing frequently-emerging infectious diseases
- Provide new solutions for better big data control of infectious diseases
- Reduce infection risk of frontline healthcare workers; create profit opportunities for clinics
- Customized multiplex point-of-care testing (POCT) —— respiratory diseases, gastrointestinal (GI) infections, sex-transmitted diseases (STDs), drug resistance, and precision oncology
Diagen has established a professional R&D and advising team, dedicated to use rapid molecular diagnosis and obtain accurate data for the patients, while the cloud system is used to store and protect patients’ data. We use special patented technique for the storage of testing reagents and the production of the chip and POCT reader, and samples can easily be obtained from saliva. Our innovations are developed using our patented technology of the integrated optoelectronics chip and the POCT reader.
Milestones & Future Plan
2022
Germination Program
- Reagent kit development
- Chip design and mold production
- Machine system integration; optimized functional testing
- Proof of concept
- Import ISO 13485 system
2023
Capstone Program & Company Founded
- Test kit design
- Outsource prototype development and production
- Complete functional performance testing for test kit; stability testing for chip and reader
- Small-scale pilot run process development
- Company founded and fund-raising
- Participated in MEDICA Trade Fair
2024
Fund-raising: Seed Round
- Preclinical trials and clinical testing
- Mass production
- Distribution channels
2025
Fund-raising: Angel Round
- Clinical evaluation
- Other respiratory diseases detection development and preclinical testing
- Domestic distribution channels (Pharmacy IVD product sales)
2026
- GI diseases detection development and preclinical testing
- Multiplex POCT reader development
- Cooperation with United States insurance company
2027
- STDs detection development
- Multiplex POCT reader optimization
2028
- Drug resistance and oncology testing
- Precision health development
- Multiplex POCT reader small-scale trial production
Risk assessment and management of aging population and multiple indications in post-pandemic era
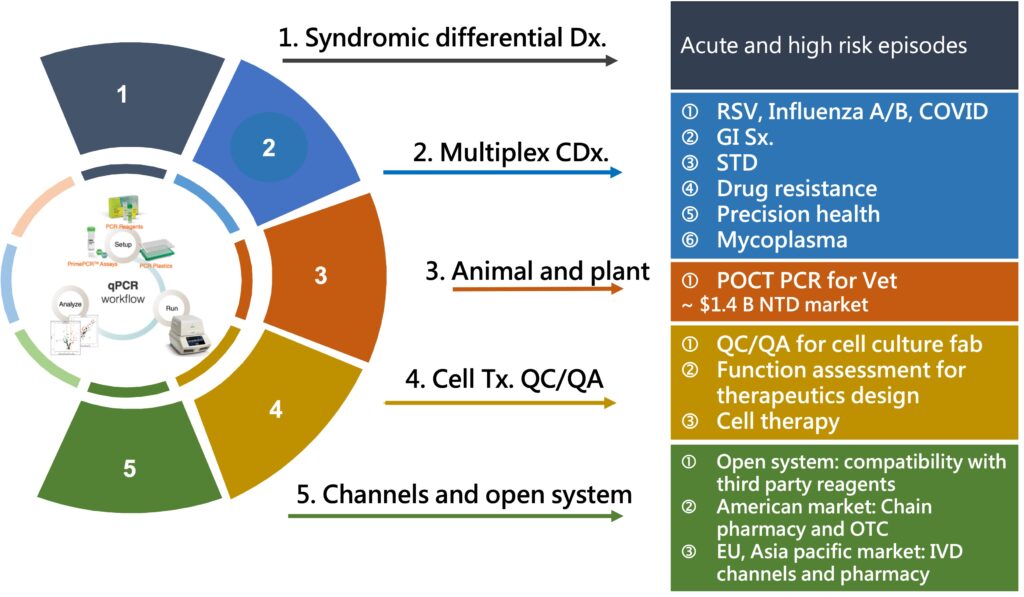
The impact of the COVID-19 epidemic has not only changed the global supply chain and industrial division of labor, but also affected life and work patterns. With the widespread application of technology, new services and business models have been derived, which will help the global economic recovery in the post-epidemic era, and bring important opportunities for digital transformation. It has also planned services for the post-epidemic era. We expect to focus on high risk groups of chronic diseases and target new market development.
